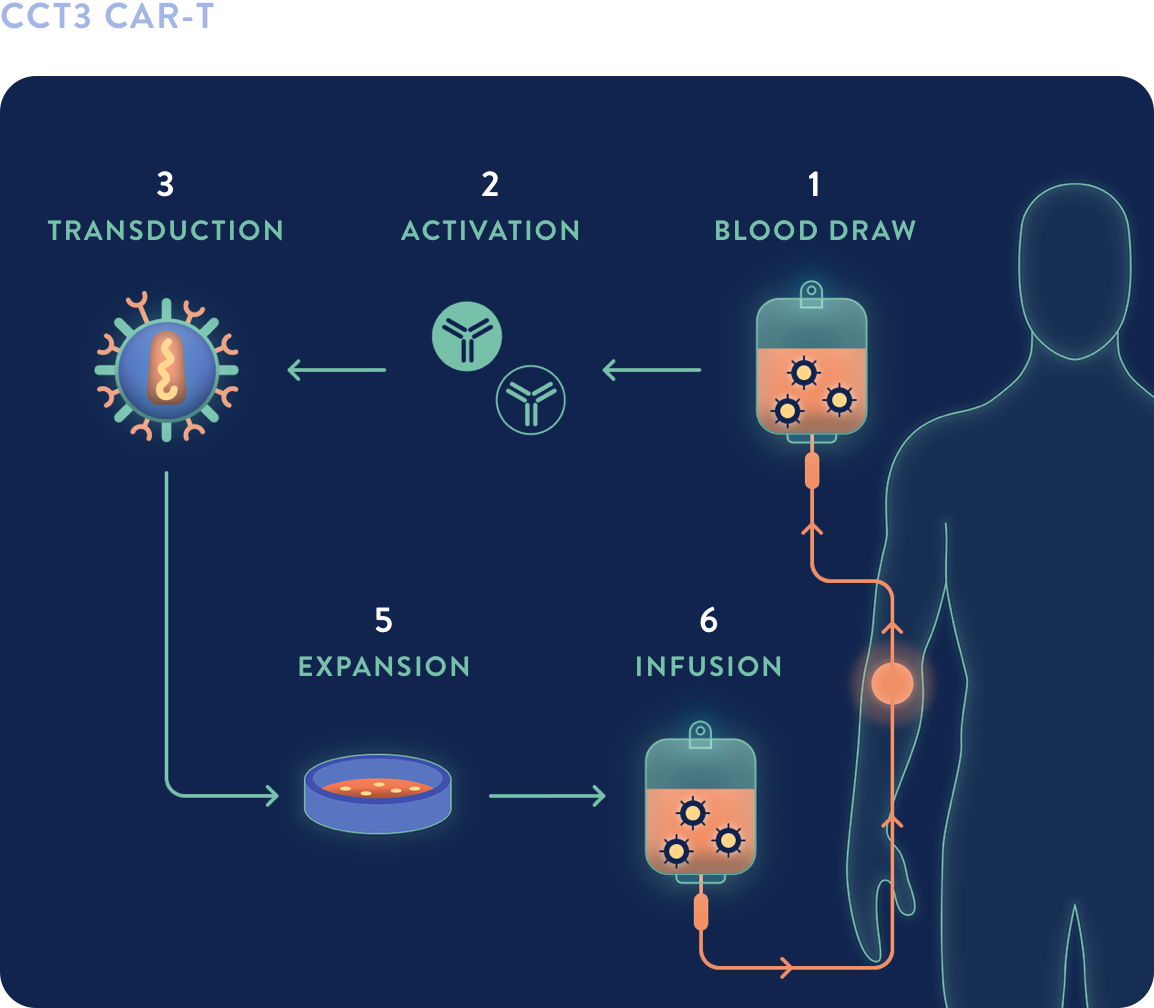
CCT3 CAR-T
EXUMA’s proprietary, centralized CCT3 CAR-T process is launch-ready and needs no further modifications to be commercially viable.
- A proprietary process which could support market entry
- 17-day vein-to-vein, fully closed, fresh in, frozen out process
- No manufacturing failures from IIT studies in China
- Uses real-time monitoring electronic batch record bar code system to help mitigate potential errors
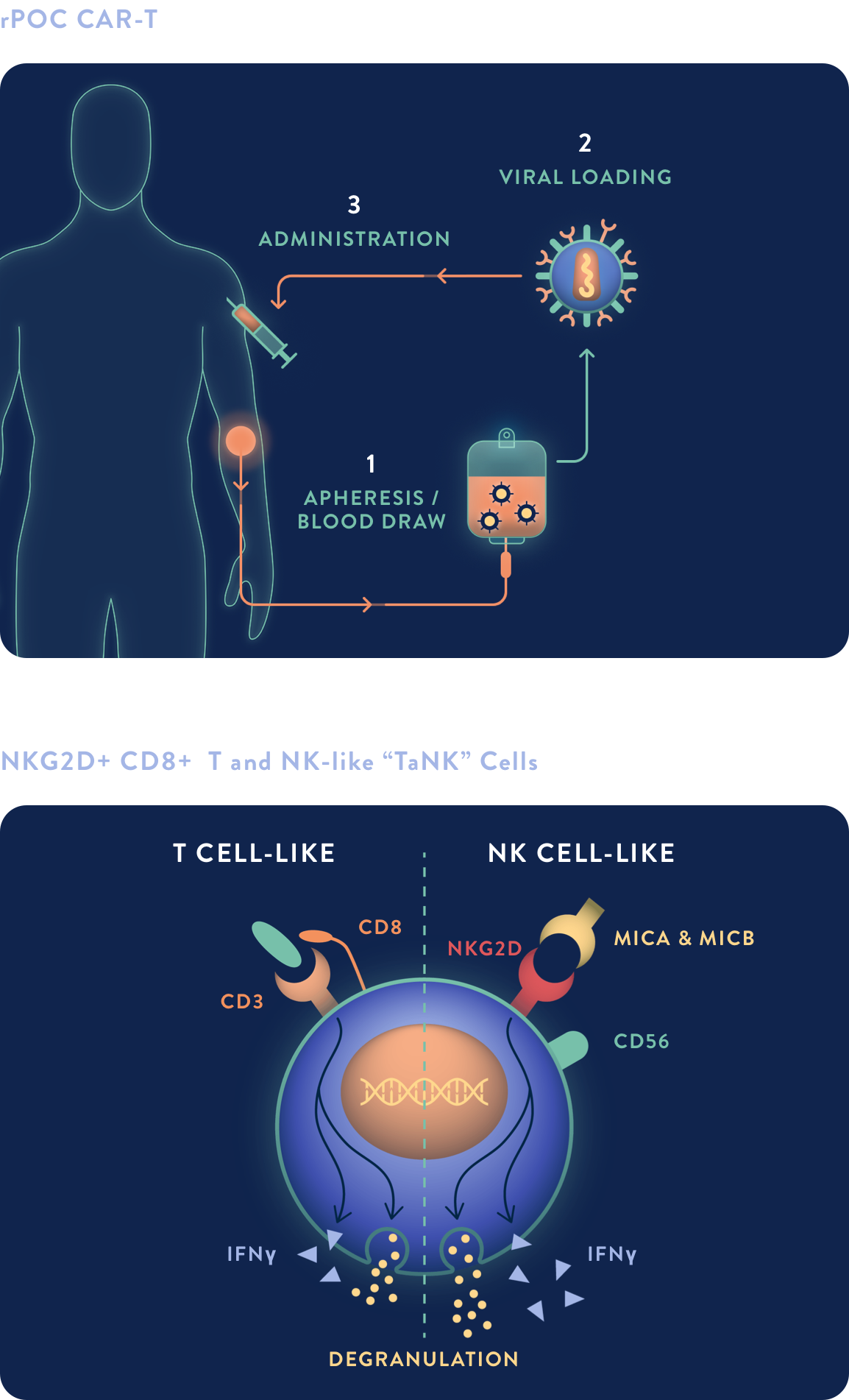
rPOC SC CAR-TaNKs
rPOC subcutaneous (SC) technology could expand access to potentially life-saving CAR-based therapies while reducing costs by avoiding the need for adjuvant chemotherapy and extended hospital admissions.
- Subcutaneously injected CAR-TaNK product can be efficiently manufactured in less than six hours
- Potential for fewer side effects by eliminating lymphodepleting chemotherapy
- Leveraging a unique T- and NK-like (“TaNK”) cell population generated from the technology, with properties of both T and NK cells, rPOC SC may produce lower systemic cytokine burden during expansion, while maintaining durability of anti-tumor response
- Has potential for administration in a broader setting of care due to potential for reduced treatment-related complications
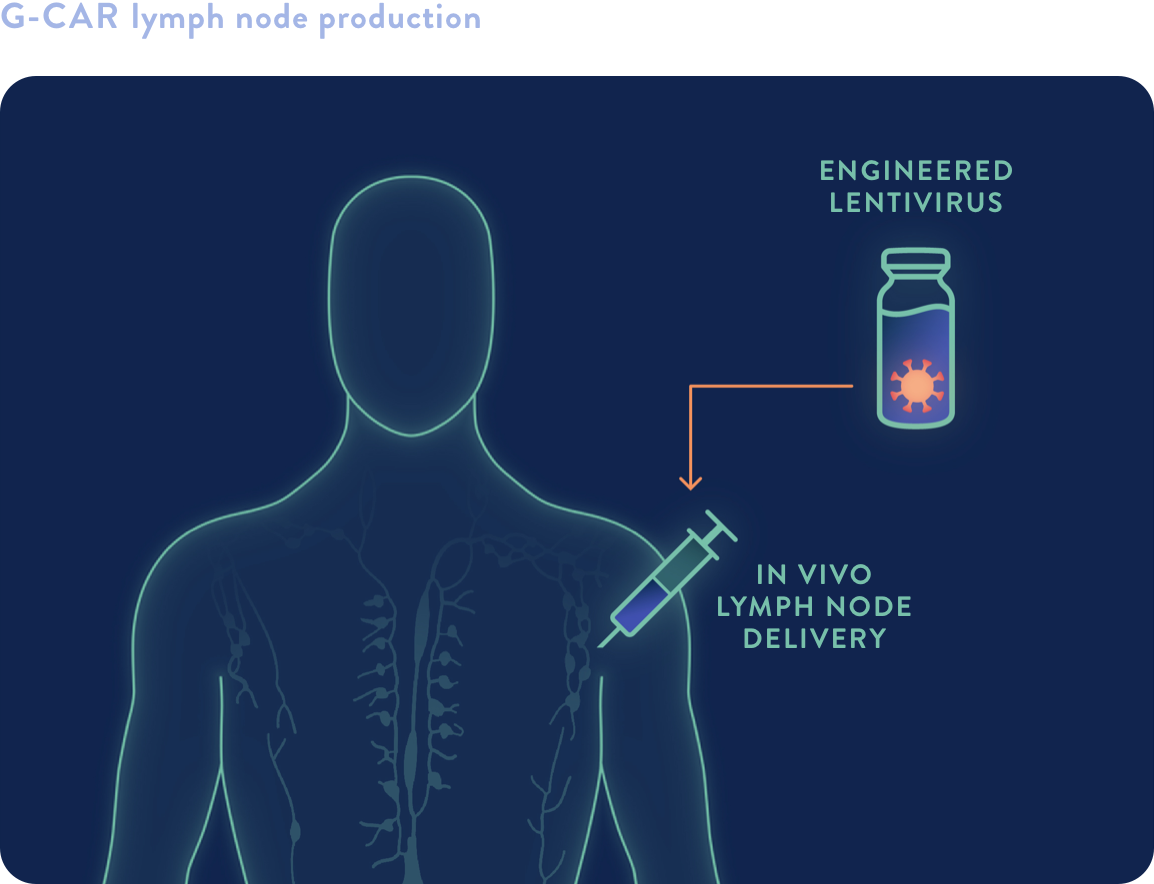
GCAR "in vivo cell therapy”
In vivo lymph node delivery of EXUMA’s off-the-shelf lentiviral vector—without lymphodepletion—for “in vivo cell therapy”
- Advancing from rPOC SC to direct injection via GCAR has the potential to accelerate cell therapy to the patient, reduce cost and complexity, and combine or sequence novel targeted therapies to achieve superior outcomes
- GCAR offers an unprecedented platform upon which to rapidly discover, test, and commercialize advanced cell therapies against hematologic and solid malignancies
- EXUMA’s viral engineering, lentiviral manufacturing, and IP portfolio are well aligned to advance this platform therapy technology