Abstract #117
Key Messages:
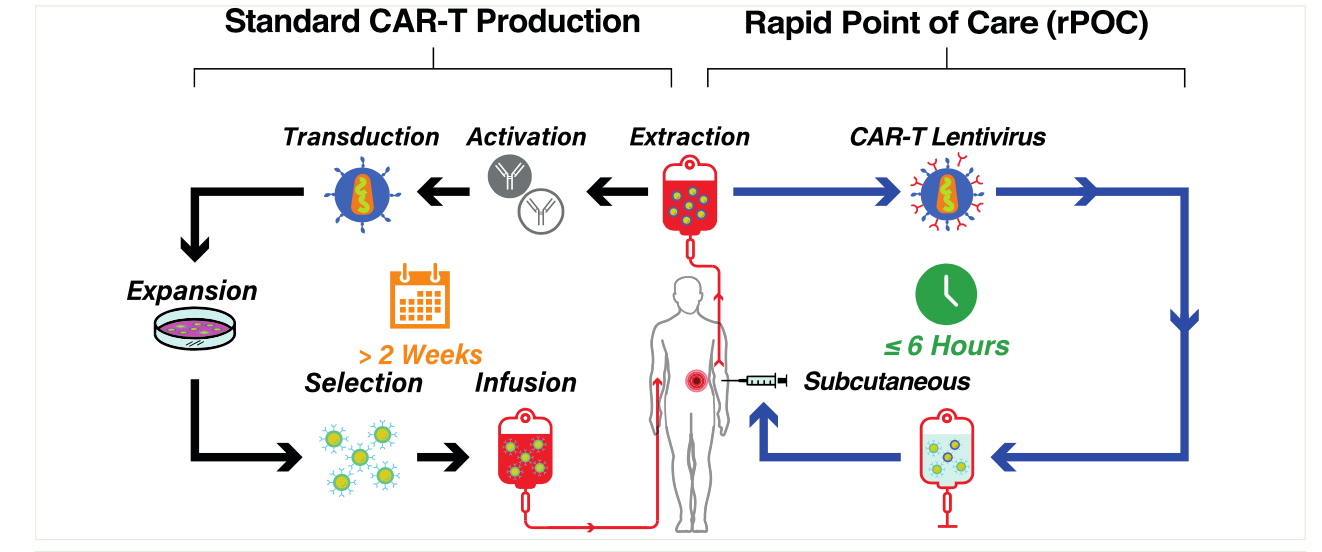
- We demonstrate that subcutaneous (SC) delivery of human PBMCs modified by a 4- hour exposure to CD3-directed lentiviruses encoding a CD19 or a CD22 Chimeric Antigen Receptor (CAR) resulted in potent anti-tumor efficacy and robust in vivo expansion of CAR-T positive cells.
- CAR-T cells driven by a synthetic domain identified from a high-throughput screening strategy exhibited > 10,000-fold expansion in vivo when given SC and demonstrated superior expansion compared to IV dosing.
- A single dose of 1 million lentiviral modified PBMC given SC resulted in complete tumor regression in subcutaneous and disseminated Raji tumor models.
- The entire process of genetic modification of PBMC and SC dosing can be completed in less than six hours, resulting in targeted genetic modification of T cells w/o prior activation. This represents a significant step forward for advancing rapid Point-of-Care (rPOC) CAR-T therapies.